Lets DIYBIO!
Well, the GFP Project has come full circle. It started out about a month ago with the idea that a few people could get together and do some science together. I would say that it has been a success. In the past few weeks we have covered what I believe to be the “Hello World” of DIYBIO, which is to transform a plasmid into a bacteria, do something with the modified bacteria, and then get the plasmid back out. Yesterday we closed the loop and extracted the plasmid.
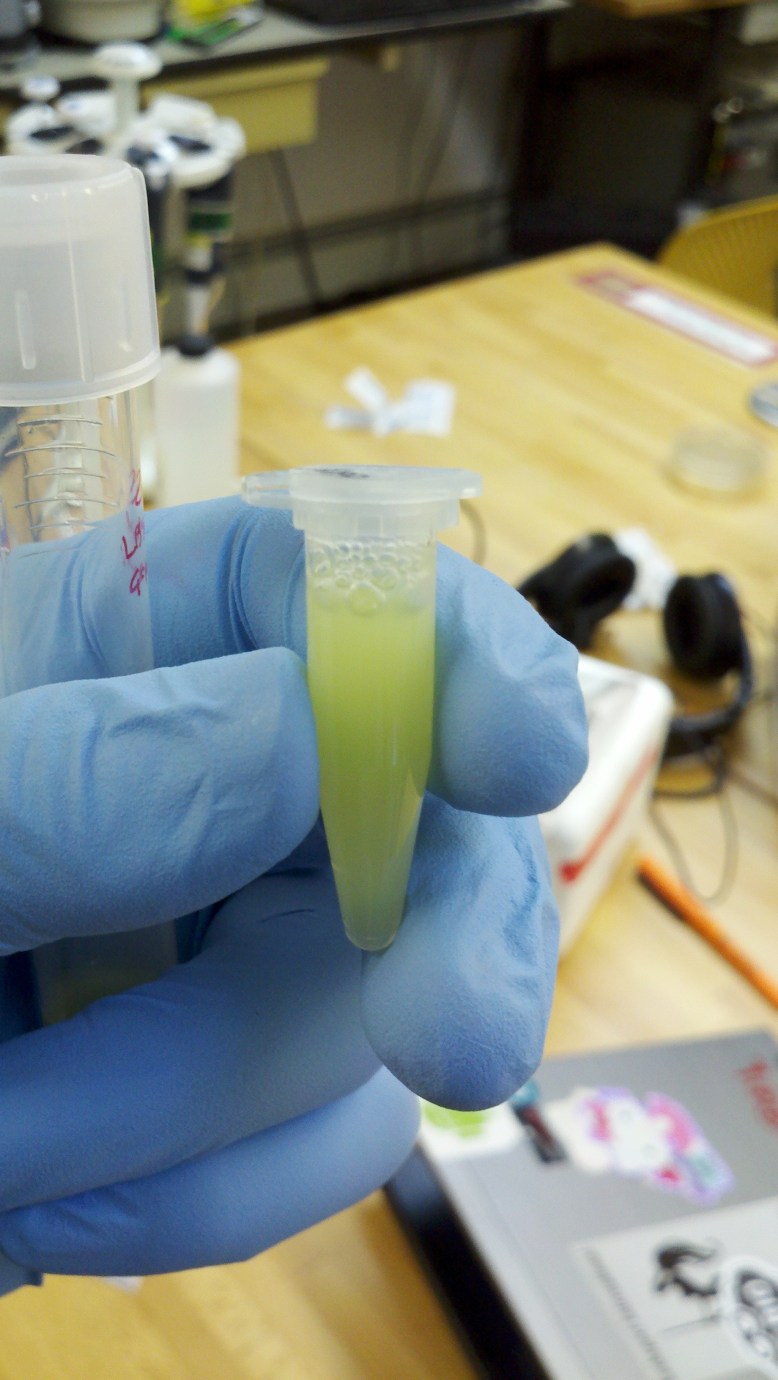
Overnight Culture
The plasmid extraction went smoothly. The Idea behind plasmid extraction is pretty simple, and it starts with an overnight culture.
Spun down cells
Then we centrifuge the tubes to pellet the cells. This allows us to pour off the supernatant, as the cells will stick to the bottom of the eppendorfs. The next step is to re-suspend the cells in “resuspension buffer”.
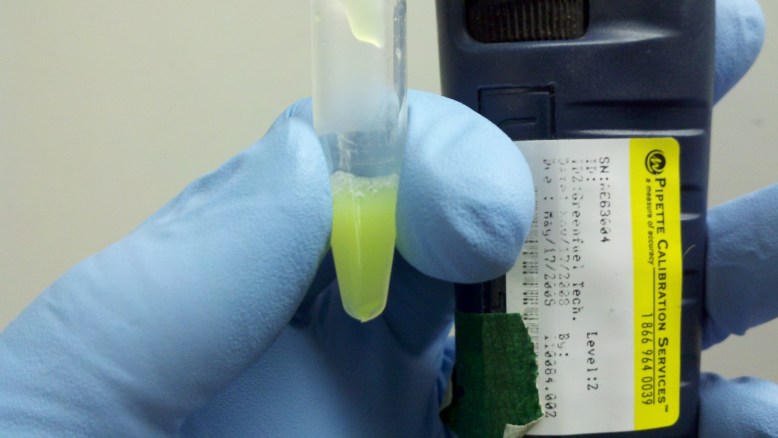
Resuspended Cells
Here are some resuspended cells! Looking pretty good. This is necessary so that the next few buffers can get to all the cells.
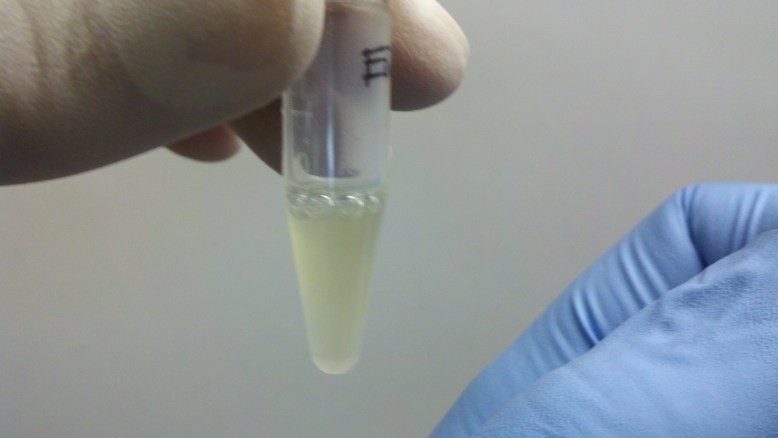
Lysed Cells
Here the cells are lysed. As you can see, the lysis buffer seems to denature the GFP, as the tube is no longer very green. The lysis allows the plasmid DNA to get out of the cell, and it also helps break down the genomic dna. The plasmid DNA is a little tougher because of its circular shape.
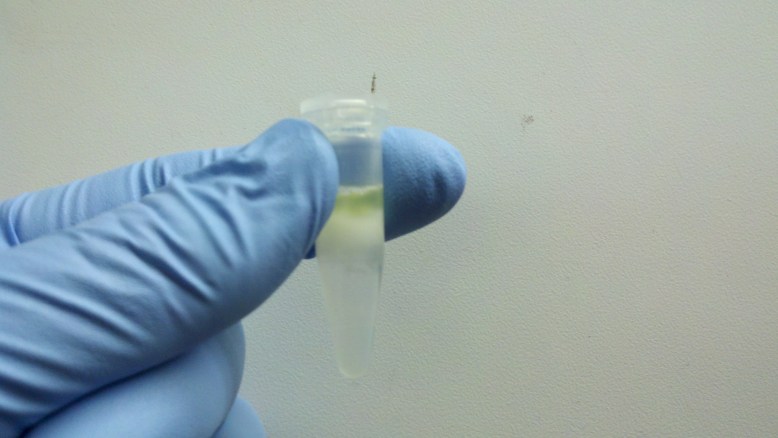
Halted Lysis
Now we halt the lysis, and this causes a change in the solubility (and probably pH and salinity), causing the extra cell ‘junk’ to fall out of solution. As you can see, the GFP has returned!
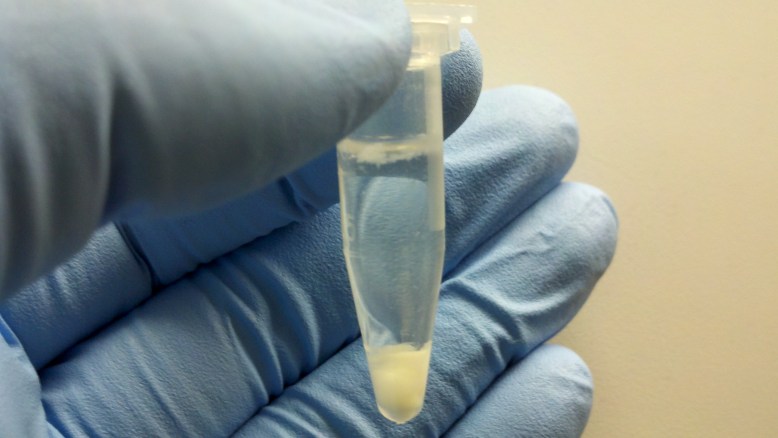
Pelleted Cell Debris
Now we pellet the cell debris in the centrifuge. This should get rid of quite a bit of the cell debris, leaving us with the plasmid DNA and some other junk in solution.
Spin Column
The supernatant (liquid) in the tube with the pelleted cell lysate is applied to the top of the spin column, and then centrifuged so that the DNA is bound to the matrix (solid white stuff) in the column. Whatever passes through is junk. To remove some of the other cell bits stuck to the column, two “wash” buffers are applied to the top of the column and centrifuged through the column. This removes other chemicals that have a negative charge like DNA, but that are not DNA.

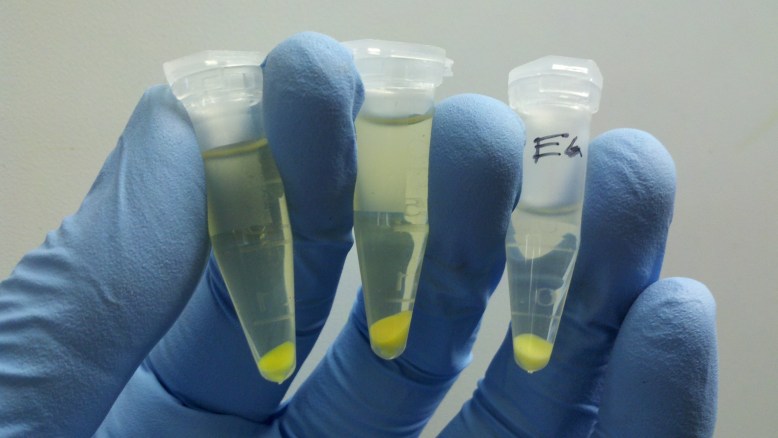
Elution!
The final step is to elute the plasmid DNA from the column by applying (you guessed it) an elution buffer. This washes the DNA out of the column. In this picture, the spin column has been placed in a clean eppendorf that will hold the final purified plasmid DNA solution.

Purified plasmid DNA
Once centrifuged, you have purified plasmid DNA!