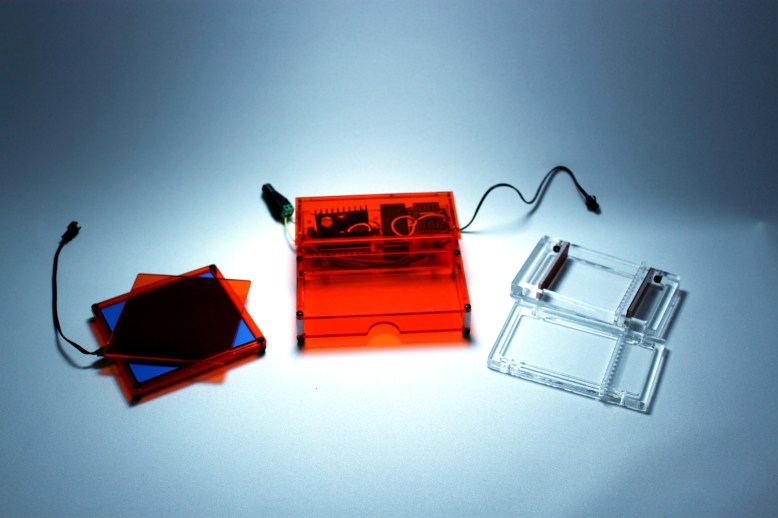
Right now I estimate that the cost breakdown of the box looks something like this:
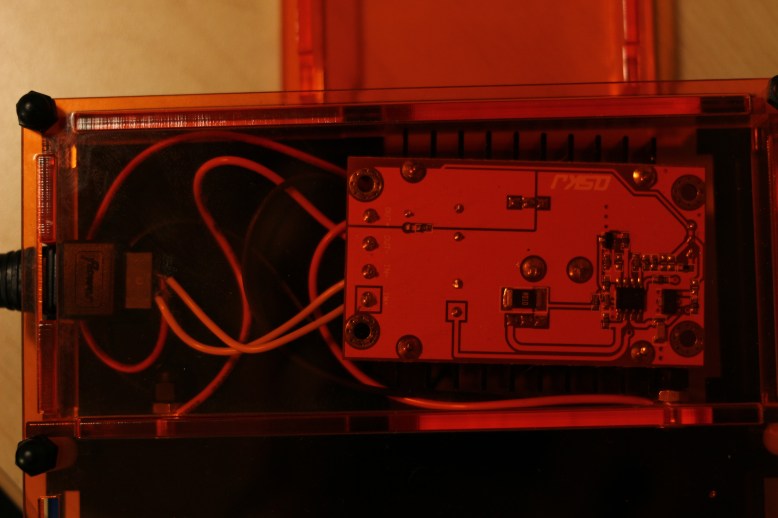
- $50 electronics
- $7 in 1/8″ plastic
- $4.75 in 1/2″ plastic
- $10 nuts bolts etc
- $24 for 12 minutes of laser time, which is currently $2 a minute
This comes out to a total cost, not including my time, of about $100. This is Kind Of A Bad Thing. I want to reduce cost, and in to do this, I am going to break the items up into three ways to cut costs. First are things that can be sourced better, like finding cheaper electronics, or finding a cheaper plastic vendor. The design might have to take into account slightly differently shaped parts, but there is not much I can do to the design to reduce cost here. The second category are things that can be reduced- using less of something or packing it in on a cut sheet tighter (using sheets of plastic more efficiently) reduces cost. The third category is reduced labor and time cost, particularly laser time. A forth category are things I am not willing to compromise on, so I didn’t include it in the enumeration of things I can do. An example of this are the nylon fasteners in the design- I love them, and they are not going anywhere.
The first category is pretty handily fixed by doing an extensive web search. I have already started this endeavor and I have ordered a few parts to see if they are high enough quality to use in the box. If I am successful, I can halve the electronics cost, bringing it down to about $25.
The second and third categories are really important to me, as a designer. It is my responsibility to reduce the amount of stuff that is used in the box, and make sure it packs nice and tight on the cutsheet. This means I need to take a hard look at what parts can be made to “stack” better on the cutsheet, and how to cut material out of the box. However, With the current design it is almost impossible to take anything more out. Most of the usage of plastic is in the walls, which can’t change in size- they have to fit the power supplies, the meters, and the gel box, and if it was made any smaller it wouldn’t do that. I have thought of alternate packing configurations, but most of them are somewhat sketchy.
That leaves the cost cutting to reducing manufacturing costs, specifically at the laser cutter. The thing that needs to be changed is the gel box itself. If you look at the breakdown of laser time, it looks like this:
- 5.5 minutes for the tray wall and floor, sometimes requiring a second pass of at least1 minute
- 1 minute for the transluminator
- 5.5 minutes for the box parts
- .5 minutes for the dams
That means in plastic cost, the WHOLE BOX including laser time is about $17. Just the gel tray costs $15.5. The gel tray also requires a post-processing step that has to be done on a drill press, which is a no-go if I want to make more than one or two (or 10. I could drill 10) of these. Given that the box itself is pretty much maxed out, it looks like I am going to have to milk the gel tray for some savings.
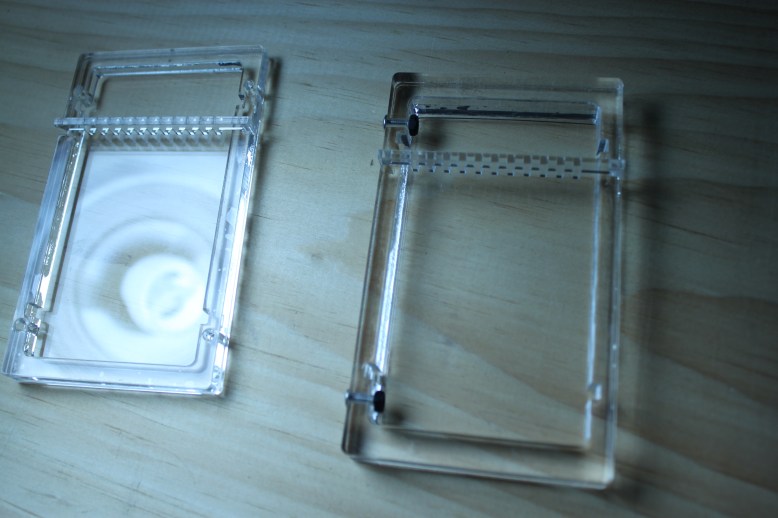
The gel tray
As I mentioned, there are some things that can’t go, like the electrodes or the dams. Some things need to go, like the holes that the screws go through in this picture. I think the answer is to look back at a past prototype.
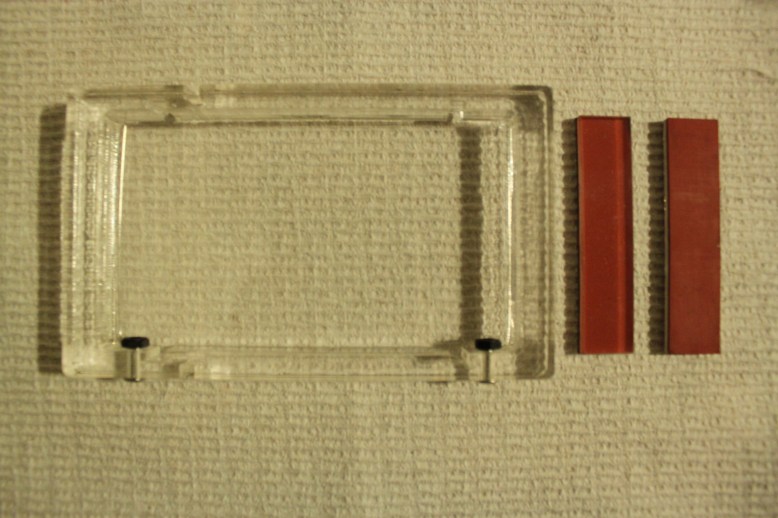
Prototype #0
This is version 0 of the gel box. There was actually a version -1, but that was so long ago I didn’t count it. This box used approximately 10ml of gel in 10 lanes, and used maybe 2ml of buffer. The goal here was to reduce the amount of material that needed to go into the gel, and make it so you could select how many lanes to run. It worked ok for things like food dye, and I even ran DNA in it once. However, there was a major problem with this box, which was the electrode choice. It was too thin (~.003″), which made it difficult to rout, and even then the rubber couldn’t seal around it, so there was really no upside. Also, bubbles would form on it, and in the thin channels, this would block the buffer from reaching the electrodes, which made the gel run unevenly.
However, it shows that you can have a really thin gel that still works. Those gels were less than .118″ thick. There have been papers published on ultra-thin gels(as thin as .01x.1×7 mm in size!, G.T. Matioli, H.B. Niewisch, Science, 150 (1965), p. 1824)), so maybe I can make it cheaper by thinning the portion that holds the gel. But to do that, I need to eliminate the holes on the side of the box (which I will be happy to do!).
So I want to try something like box 0, in that it is shallow, and like box 2, in that it is functional. To get rid of the holes in the side, I am going to add in-cut plane holes for bolts, just like in box 0 for the banana plugs. These will go to metal screws that will probably go through the box, into one of my favorite fasteners of all time, the 6/6 nylon 4-40 acorn nut. These acorn nuts will slip into holes in the bottom of the gel tray so you know the tray is in the right place and they will help keep it there. Of course, there will be a small grove on the top and bottom of the box (the part that holds the tray) so you can grab the side of the tray out, but the fit will be tight so there is little chance of electrocution (as small as you can expect with a regular gel box).
Even if this only cuts the cost of the tray in half laser time-wise, it is still a simpler, better way to build the gel tray, and the plastic to build it is more readily available and much cheaper. Since it is cheaper/better, it will make it cheaper to have more trays, which means you can save your gels/precast your gels, and not have to worry! Look for this in prototype #3!